Ammoniacal Nitrogen
Home > WWT Tech > Ammoniacal Nitrogen
Ammoniacal Nitrogen Treatment
Amines, Amides and Free Ammonia
Ammoniacal Nitrogen
Why is Ammoniacal Nitrogen an Issue?
Are you currently experiencing any Ammoniacal Nitrogen issues in your effluent or your existing ETP?
When transitioning from liquid to gas, various forms of compounded Nitrogen molecules can be highly toxic to humans as atmospheric emissions
The presence of Nitrogenous organic matter, specifically Ammoniacal Nitrogen,can disturb the ecological balance of microorganisms present in biologically driven ETPs. Such organic matter tends to consume most of the available oxygen, thereby leaving lesser oxygen for COD degradation.
Due to the differing boiling points of nitrogenous organic compounds, some of them may skip the stripping stage and enter the MEE/MVRE Condensate, resulting in the condensate being unusable in its current state
Loosely bonded nitrogenous organic compounds, if not entirely degraded, can react with other available matter in ETPs, forming transient compounds that further complicate the fluid’s chemistry to be treated.

What is the impact of Boson Alchemies MHO Forced Oxidation Systems on
Ammoniacal Nitrogen?
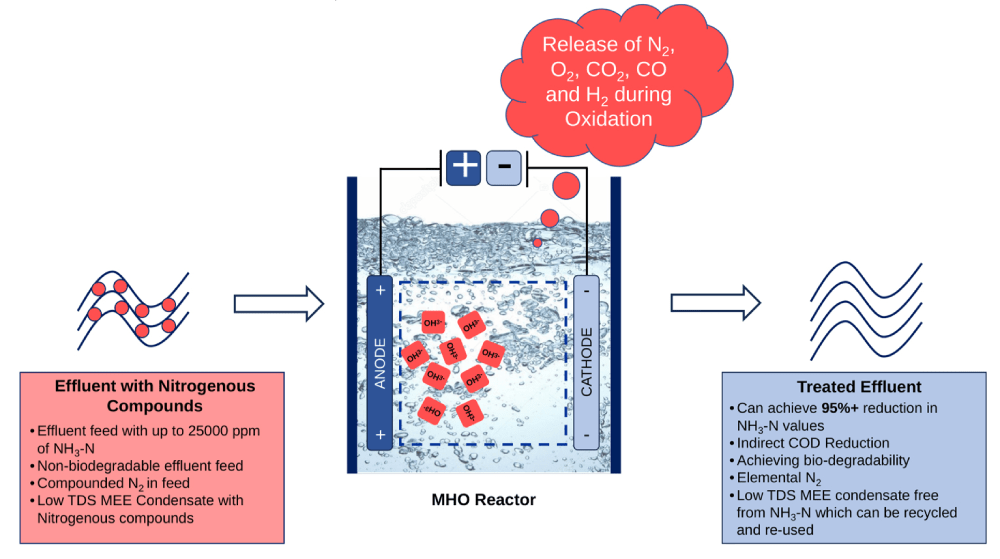
Boson Alchemies has engineered a wide assortment of catalysts. For NH3-N treatment, a unique grade of catalyst has been developed with the ability to immobilize nitrogenous compounds once released into the fluid. Following immobilization, a complementary catalyst forms ligands around the nitrogenous compounds, which become highly receptive to OH3- (a high potential oxidative radical) released by another set of catalysts. The result is the oxidative breakdown of the nitrogenous compounds into simpler elements and compounds such as N2, O2, CO2, and H2.
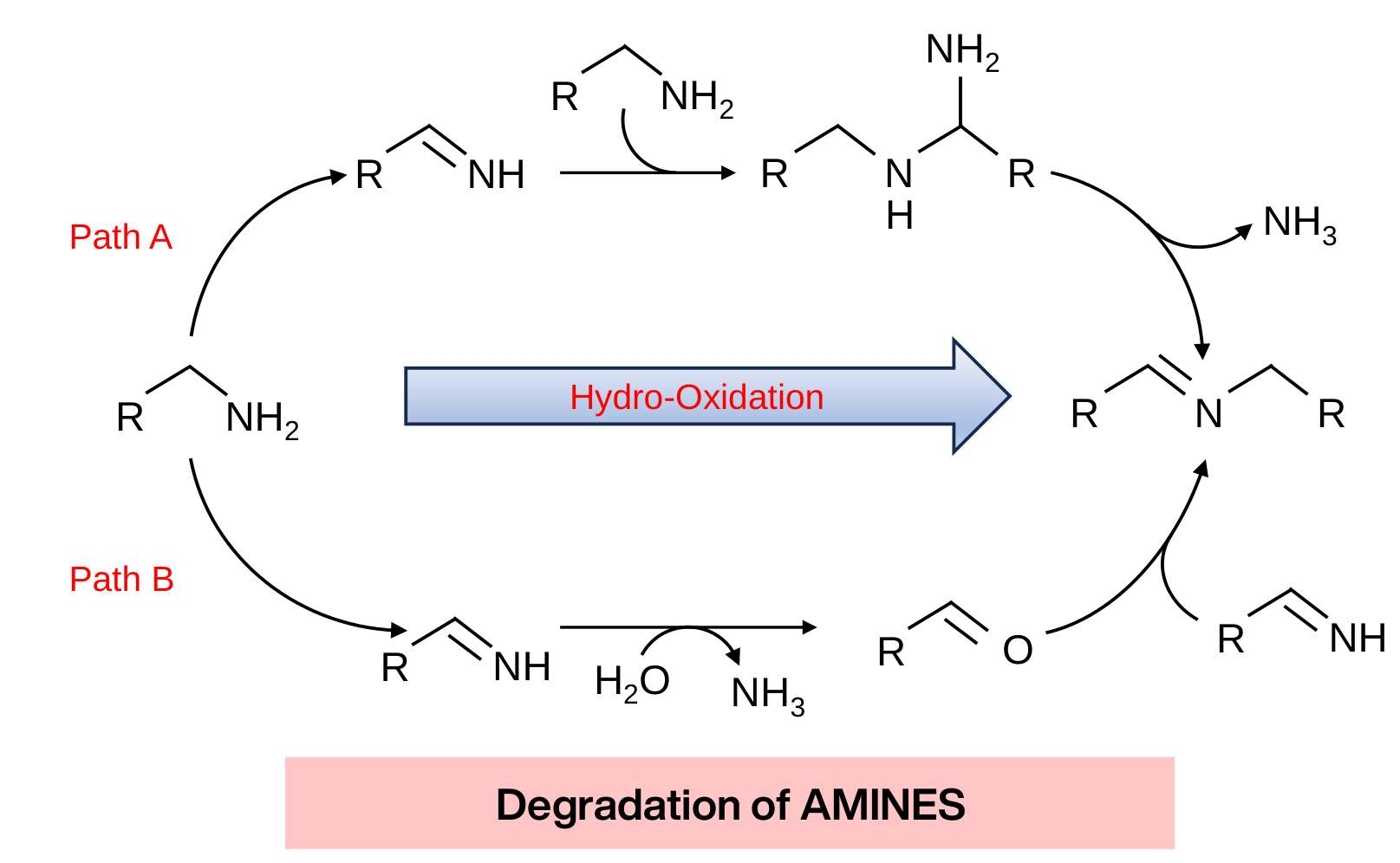
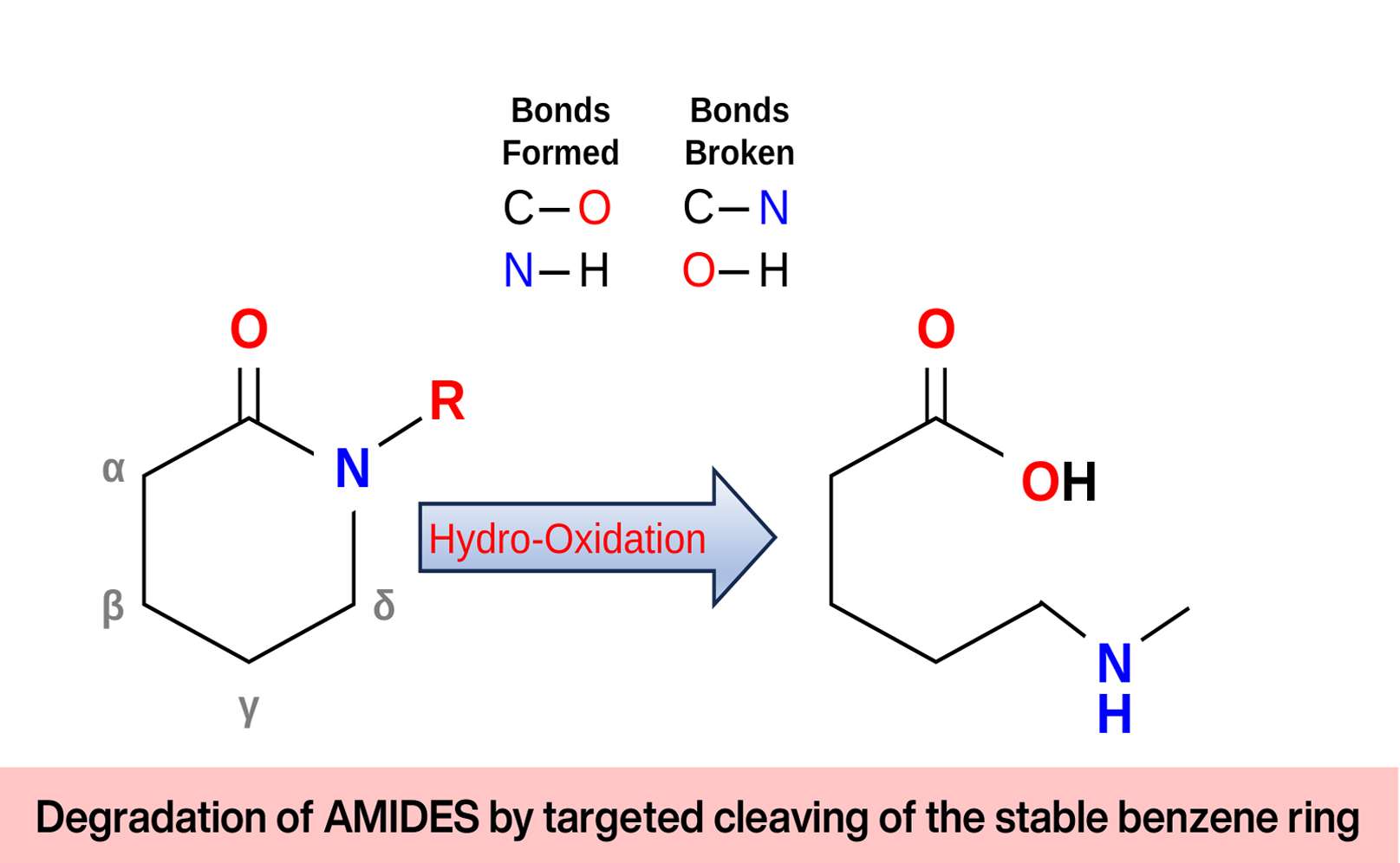
This methodology targets nitrogenous compounds, including amines, amides, and imines, for comprehensive oxidation-degradation.
Benefits
Complete degradation of nitrogenous compounds to simpler elements / compounds such as N2, O2, CO2, H2 etc

Enables consistency of feed acceptable feed into downstream biological ETP

Avoids phase change facilitated air pollution

These systems can completely make nitrification & de-nitrification process obsolete
Process Flow Diagram
Target Industry

Specialty Dyes, Pigments & Paints (Paper Dyes included)

Pharmaceuticals / API Manufacturing

Food & Beverage Industry

Hydrocarbon processing Industries

Specialty Chemicals
(Largely aromatics – perfumes & essence)
Capacity & Footprint
MHO oxidation reactors utilized for Ammoniacal Nitrogen removal are designed to be compact, given that the residence/treatment time ranges from 10 to 60 minutes.
Typical wastewater treatment systems are unable to process effluents with elevated levels of ammoniacal nitrogen as the MLSS (mixed liquor suspended solids) cannot thrive in such conditions, thus rendering any size comparison moot
Compared to conventional systems, our MHO reactor installations require significantly less space – less than 10% – and can be easily installed as multiple modules for capacities exceeding 100 KLD.
